


Chemistry: Energy changes
What you need to know
Reflections and Exam tips
Energy changes
Representing energy changes
The energy changes in a chemical reaction can be conveniently represented using energy level diagrams.
Energy level diagrams make it easier to decide whether a reaction is exothermic (gives out heat and gets hotter) or endothermic (takes in heat and gets cooler).
Measuring enthalpy changes
To determine the amount of heat a substance produces or absorbs we often use q = cmT. Where C is the specific heat capacity, m is the mass of solution and delta T is change in temperature.
Specific heat capacity = heat capacity of 1 g of a substance. It takes 4.2 J to heat 1 ml/g of solution by 1 °C, so specific heat capacity for water is 4.2 J/ml/°C.
To determine the enthalpy change of the reaction the following reaction is used.
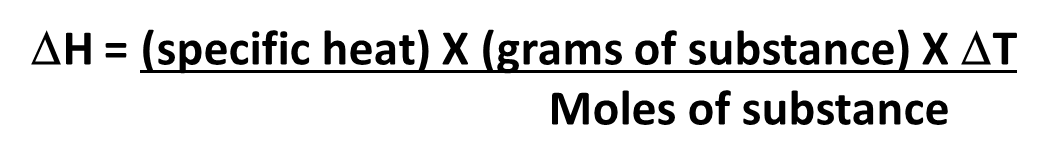
Bond energies
Bond Energy- energy required to break apart a bond between two particular atoms, measured in kJ/mol and used to work out ΔH in energy calculations.
Chemical reactions are in two stages:
- Breaking bonds, an endothermic process.
- Making new bonds, an exothermic process.
To calculate energy change we need to know:
a) the amount of energy needed to break the bonds between the atoms;
b) the amount of energy released in the formation of new chemical bonds.
Consider the reaction between hydrogen and chlorine:
H2 + Cl2 ——> 2HCl
In this reaction one H-H bond and one Cl-Cl bond is broken and two H-Cl bonds are formed.
The H-H bond energy is 436kJ/mol
The Cl-Cl bond energy is 242kJ/mol
So, the energy needed to break these bonds is 436 + 242 = 678kJ
The H-Cl bond energy is 431kJ
So the energy given out when these bonds are
formed is 2 x -431 = -862kJ
Overall change = 678 + (– 862) = -184kJ, an exothermic reaction.